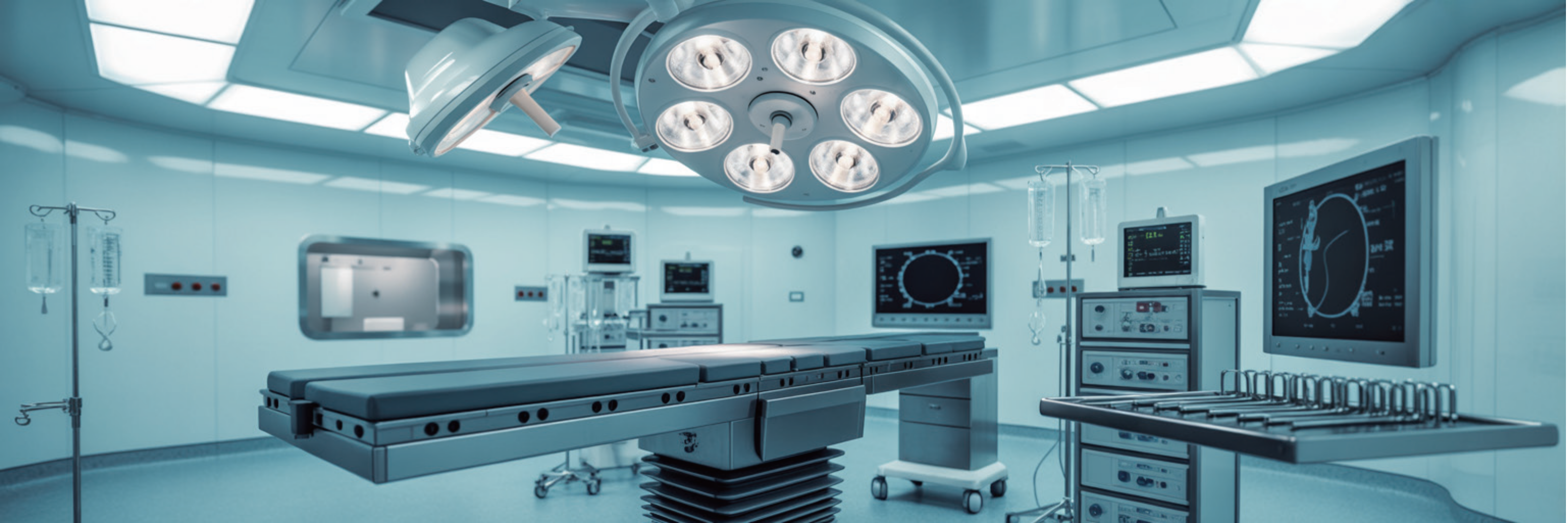
With over 30 years of medical industry experience and complete ISO 13485 certification, CTi specialises in small gauge, bio-compatible, bio-burden controlled and application-driven medical device cables and tubing. Our vertical integration allows us to co-develop and manufacture custom solutions for major medical device companies around the world.
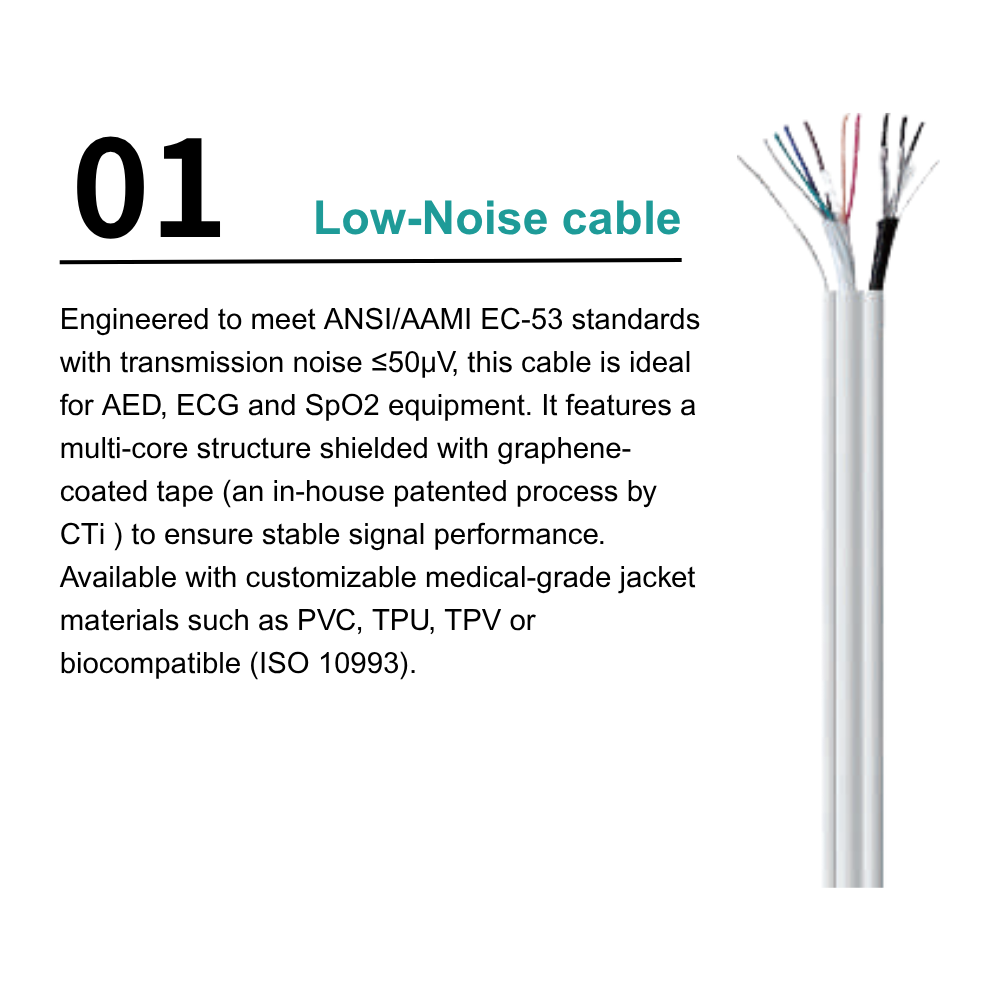
Product Examples



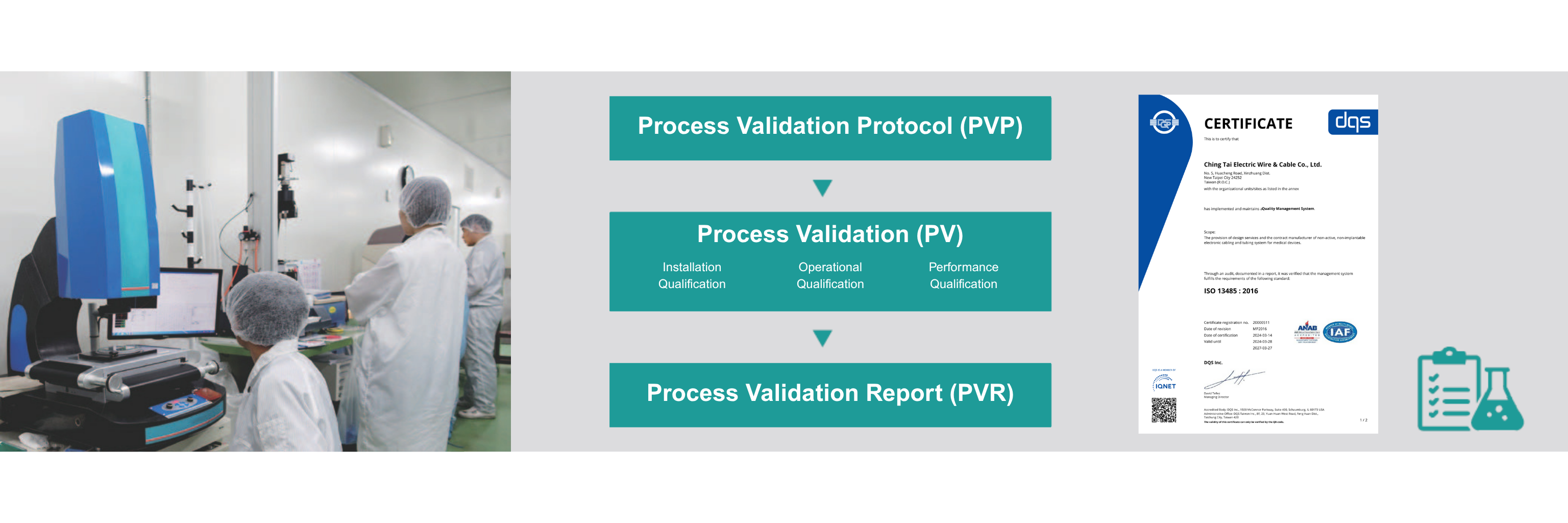
CTi holds ISO 9001, ISO 13485, ISO 14001, and ISO 14064 certifications as part of our quality system.Our NEBB-certified Class 100K cleanroom facility supports medical device cable and tubing manufacturing.Process Validation (PV) is conducted in accordance with a clearly defined Process Validation Protocol (PVP), encompassing equipment, personnel, and inspection methods. Upon completion, a comprehensive Process Validation Report (PVR) is compiled, detailing procurement records, measurement data, and process history — all reviewed and approved by the relevant departments.
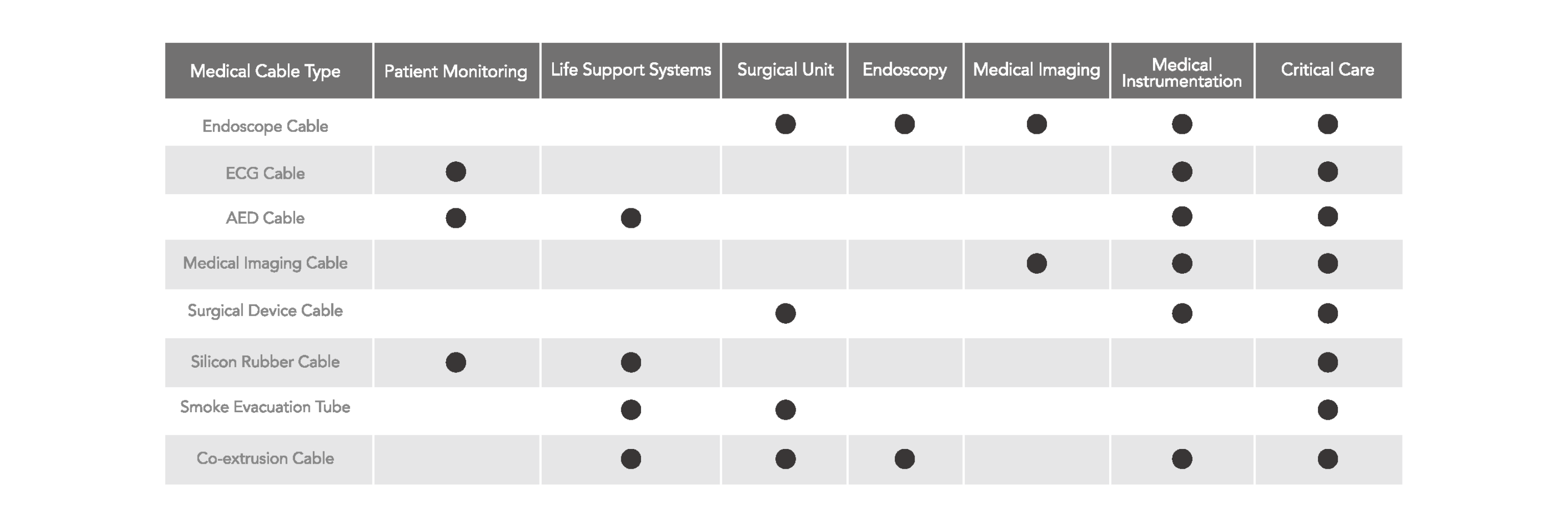
Applications
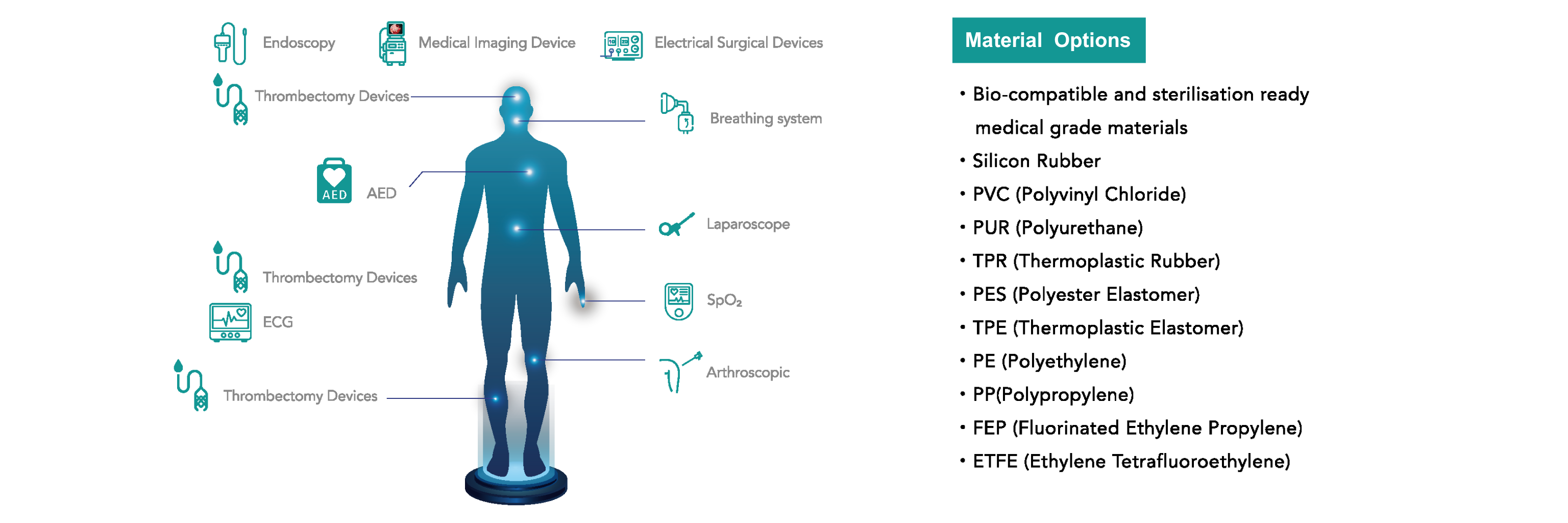
We offer custom medical cables and tubing to meet your specific application needs.






 256 bit SSL Encryption
256 bit SSL Encryption